扎那米韦吸入粉雾剂
一、药品说明书
【药品名称】
通用名:扎那米韦吸入粉雾剂
商品名:依乐韦®、RELENZA®、瑞乐沙®、乐感清®、也青®
英文名称:Zanamivir Powder for Inhalation
汉语拼音:ZaNaMiWei XiRu Fenwuji
化学结构式: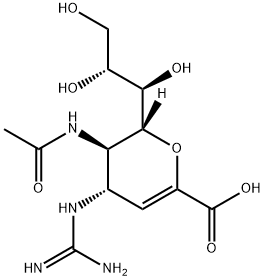
分子式:C12H20N4O2
分子量:332.3
【成分】本品主要成份扎那米韦。
【性状】本品为胶囊型吸入粉雾剂,内容物为白色或类白色粉末。
【适应症】
用于成人和7岁及7岁以上儿童的甲型和乙型流感治疗。
治疗应尽早开始,且不应晚于感染初始症状出现后48小时(见【注意事项】)。
治疗甲型和乙型流感时,抗病毒药物非必需使用的药物,因此使用本品治疗流感时,应慎重考虑其必要性。
【规格】5mg/泡(以扎那米韦计)
【用法用量】、【不良反应】、【禁忌】、【注意事项】、【孕妇及哺乳期妇女用药】、【儿童用药】、【老年用药】、【药物相互作用】、【药物过量】、【药理毒理】、【药代动力学】详细内容见说明书。
【贮藏】置于阴凉处/30℃以下贮存。
【包装】扎那米韦吸入粉雾剂由10粒/板×2板的铝塑泡罩装胶囊,外加一个旋转式吸入器组成。使用时需采用旋转式吸入器经口吸入给药。
【有效期】24个月/36个月/84个月
【执行标准】YBH00342010、JX20060094
【批准文号】国药准字H20163314、国药准字H20103037、国药准字H20100011
【生产企业】山东新时代药业有限公司(原料药)、先声药业有限公司、GlaxoSmithKline Australia Pty Ltd(排名无先后顺序)
【说明书】
1.南京先声东元制药有限公司
链接:https://zy.yaozh.com/instruct/20180606sms/a343.pdf、https://zy.yaozh.com/instruct/sms20220809/wh20223300937.pdf
2.GlaxoSmithKline Australia Pty Ltd
链接:https://zy.yaozh.com/instruct/20160422/54.jpg
二、信息查询
1.国家药品监督管理局(NMPA)
链接:https://www.nmpa.gov.cn/datasearch/search-result.html
2.欧洲药品管理局(EMA)
链接:https://www.ema.europa.eu/en/medicines?search_api_views_fulltext=Zanamivir
3.美国药品信息查询(FDA)
链接:https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process
4.日本药品信息查询(PMDA)
链接:https://www.pmda.go.jp/PmdaSearch/iyakuSearch
三、指南或专家共识
1.四川省流行性感冒中西医结合诊疗专家共识(2023版)
链接:https://guide.medlive.cn/guideline/28355
2.流行性感冒诊疗方案(2020年版)
链接:https://guide.medlive.cn/guideline/21925
3.2022-2023 AAP儿童流感的预防与控制建议
链接:https://publications.aap.org/pediatrics/article/150/4/e2022059274/189385/Recommendations-for-Prevention-and-Control-of?autologincheck=redirected
4.2022瑞典指南:流感的管理——抗病毒治疗(更新)
链接:https://guide.medlive.cn/guideline/29320
5.2022-23 ACIP建议:流感季节流感疫苗防控
链接:https://www.cdc.gov/mmwr/volumes/71/rr/rr7101a1.htm?s_cid=rr7101a1_w
6.成人流行性感冒诊疗规范急诊专家共识(2022版)
链接:https://guide.medlive.cn/guideline/27512
四、药物动力学/药效学
1.扎那米韦在接受连续静脉血液滤过的危重患者中的药代动力学
(Pharmacokinetics of zanamivir in critically ill patients undergoing continuous venovenous hemofiltration)
作者:André Wieringa(Department of Clinical Pharmacy, 8772Isala Hospital, The Netherlands)
链接:https://pubmed.ncbi.nlm.nih.gov/36609161/
2.扎那米韦-胆固醇偶联物:一种对耐药性流感病毒具有强效作用的长效神经氨酸酶抑制剂
(Zanamivir-Cholesterol Conjugate: A Long-Acting Neuraminidase Inhibitor with Potent Efficacy against Drug-Resistant Influenza Viruses)
作者:Xun Lv(CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences (CAS), China)
链接:https://pubmed.ncbi.nlm.nih.gov/34797984/
3.吸入型抗病毒药物临床药代动力学的系统评价
(A Systematic Review of Clinical Pharmacokinetics of Inhaled Antiviral)
作者:Mohammed Kanan Alshammari(Department of Clinical Pharmacy, King Fahad Medical City, Arabia)
链接:https://pubmed.ncbi.nlm.nih.gov/37109600/
五、临床研究
1.扎那米韦联合豉翘清热颗粒在儿童急性上呼吸道感染治疗中的应用价值及疗效影响因素研究
作者:张长玲(西南医科大学附属医院儿科,四川)
链接:https://d.wanfangdata.com.cn/periodical/ChhQZXJpb2RpY2FsQ0hJTmV3MjAyMzA4MDYSDnp5eXhrMjAyMDExMDUyGghkdWxtN2hidg%3D%3D
2.喜炎平注射液联合扎那米韦治疗甲型H1N1流感的临床研究
作者:冯晓敏
链接:https://d.wanfangdata.com.cn/periodical/ChhQZXJpb2RpY2FsQ0hJTmV3MjAyMzA4MDYSDWJmeXgyMDE3MDIxMDMaCGx3eW4xN2Zq
3.扎那米韦治疗流感的五天疗程,使用单一、自我给药、无痛的微针阵列贴片:对膜渗透性差的治疗药物的革命性递送
(A five-day treatment course of zanamivir for the flu with a single, self-administered, painless microneedle array patch: Revolutionizing delivery of poorly membrane-permeable therapeutics)
链接:https://pubmed.ncbi.nlm.nih.gov/37230371/
作者:Dawn Reyna(TSRL, Inc., 540 Avis Dr., Suite A, Ann Arbor, USA.)
4.通过同情静脉注射扎那米韦成功治疗复杂性甲型H3N2流感病毒感染和横纹肌溶解症
(Successful Treatment of Complicated Influenza A(H3N2) Virus Infection and Rhabdomyolysis with Compassionate Use of IV Zanamivir)
作者:Maren Alchikh(Vaccine Safety Initiative, Germany)
链接:https://pubmed.ncbi.nlm.nih.gov/36678583/
5.Alloferon和扎那米韦在体外和体内对甲型流感病毒(H1N1)感染显示出有效的抗病毒活性
(Alloferon and Zanamivir Show Effective Antiviral Activity against Influenza A Virus (H1N1) Infection In Vitro and In Vivo)
作者:Dahae Lee(Laboratory of Vitamin C and Antioxidant Immunology, Department of Anatomy and Cell Biology, Seoul National University College of Medicine, Republic of Korea)
链接:https://pubmed.ncbi.nlm.nih.gov/36614125/
6.静脉注射扎那米韦临床试验的耐药性分析教训
(Lessons from resistance analysis in clinical trials of IV zanamivir)
作者:Phillip J Yates(GlaxoSmithKline, UK)
链接:https://pubmed.ncbi.nlm.nih.gov/36610656/
7.计算机研究表明,即使H7N9禽流感病毒获得进一步耐药性,帕拉米韦和扎那米韦也是最佳药物治疗药物
(In Silico Studies Reveal Peramivir and Zanamivir as an Optimal Drug Treatment Even If H7N9 Avian Type Influenza Virus Acquires Further Resistance)
作者:Edita Sarukhanyan(Department of Bioinformatics, Biocenter, Am Hubland, University of Würzburg, Germany)
链接:https://pubmed.ncbi.nlm.nih.gov/36144655/
8.吸入扎那米韦与口服奥司他韦在预防流感相关住院或死亡方面的比较:一项基于全国人群的类实验性研究
(Inhaled Zanamivir vs Oral Oseltamivir to Prevent Influenza-related Hospitalization or Death: A Nationwide Population-based Quasi-experimental Study)
作者:Chia Ping Su(Taiwan Centers for Disease Control, Ministry of Health and Welfare, China)
链接:https://pubmed.ncbi.nlm.nih.gov/35299245/
9.神经氨酸酶抑制剂在流感患者中的疗效比较:一项系统综述和网络荟萃分析
(Comparative effectiveness of neuraminidase inhibitors in patients with influenza: A systematic review and network meta-analysis)
作者:Hui-Chen Su(Department of Pharmacy, Chi Mei Medical Center, China)
链接:https://pubmed.ncbi.nlm.nih.gov/34840038/
10.静脉注射扎那米韦:危重流感患者的可行选择
(Intravenous Zanamivir: A Viable Option for Critically Ill Patients With Influenza)
作者:Douglas Slain(West Virginia University, USA)
链接:https://pubmed.ncbi.nlm.nih.gov/33016090/
六、不良反应
1.过敏反应与抗流感药物使用之间的关联:日本药物不良事件报告数据库分析
(Association between anaphylaxis and anti-influenza drug use: An analysis of the Japanese Adverse Drug Event Report database)
作者:Hiroyuki Tanaka(Department of Practical Pharmacy, Faculty of Pharmaceutical Sciences, Toho University, Japan)
链接:https://pubmed.ncbi.nlm.nih.gov/34234064/
2.使用FDA不良事件报告系统和在线患者评论评估研究抗流感神经氨酸酶抑制剂相关的不良事件
(Assessment of adverse events related to anti-influenza neuraminidase inhibitors using the FDA adverse event reporting system and online patient reviews)
作者:Nayoung Han (College of Pharmacy and Research Institute of Pharmaceutical Sciences, Seoul National University, Republic of Korea)
链接:https://pubmed.ncbi.nlm.nih.gov/32080337/


 京公网安备11010502042549号
京公网安备11010502042549号